Reblozyl
What is Reblozyl (Luspatercept)?
Approved To Treat
Top Global Experts
Related Clinical Trials
Summary: This is a Phase 2a study to evaluate the safety and pharmacokinetics (PK) of luspatercept in pediatric participants with β-thalassemia. The study will be conducted in 2 parts for both transfusion-dependent (TD) and non-transfusion-dependent (NTD) β-thalassemia participants: TD Part A will be in adolescent participants aged 12 to \<18 years with two dose escalation cohorts, followed by a dose expan...
Summary: The purpose of the study is to compare the efficacy and safety of Luspatercept vs epoetin alfa in the treatment of anemia in adults due to IPSS-R very low, low, intermediate-risk MDS in ESA-naïve participants who are non-transfusion dependent (NTD).
Summary: The purpose of this Phase 2 study is to evaluate the efficacy and safety of momelotinib (MMB) in combination with luspatercept (LUSPA) in participants with transfusion dependence (TD) primary myelofibrosis (PMF) or Post-polycythemia vera (PV)/ essential thrombocythemia (ET) myelofibrosis (MF) who are either janus kinase (JAK) inhibitor (JAKi) naïve or experienced.
Related Latest Advances
Brand Information
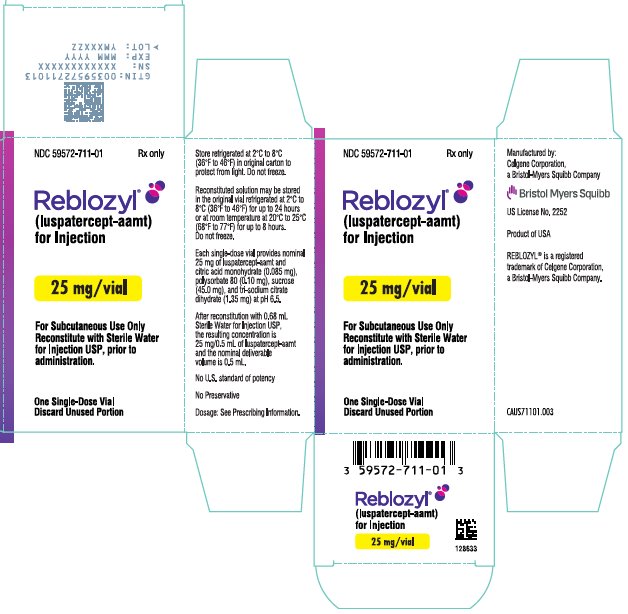
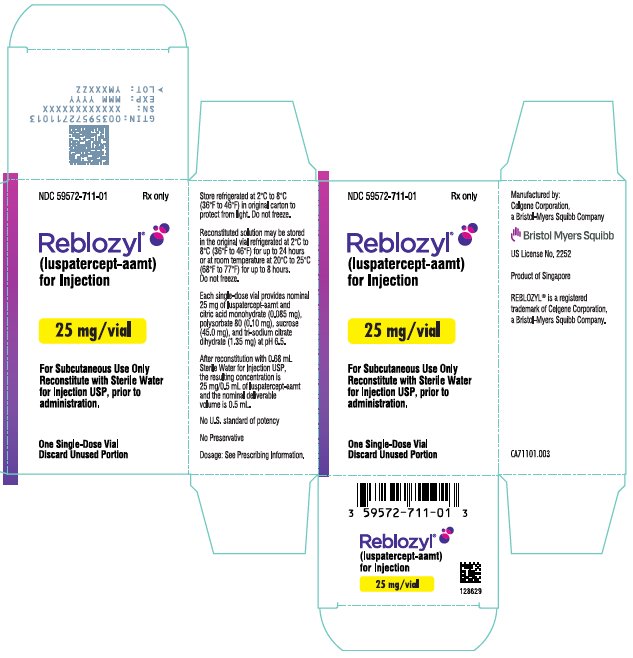
- For injection: 25 mg white to off-white lyophilized powder in a single-dose vial for reconstitution.
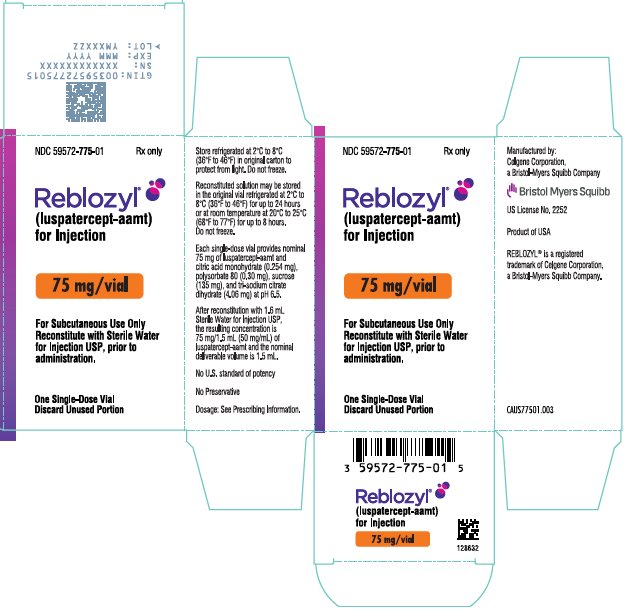
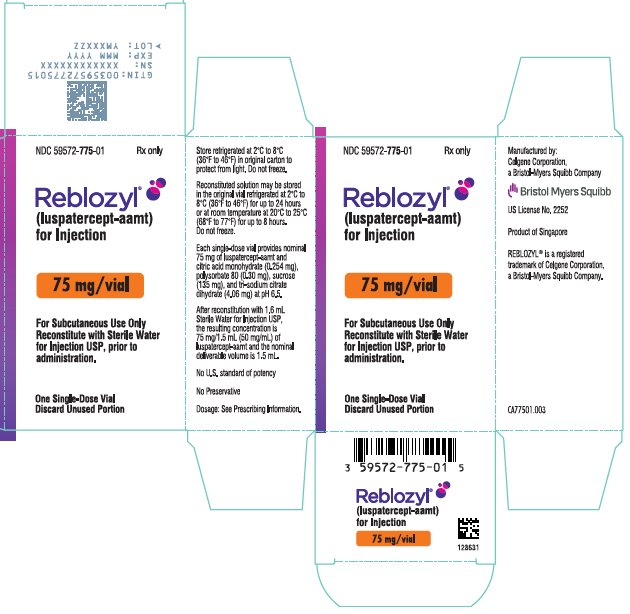
- For injection: 75 mg white to off-white lyophilized powder in a single-dose vial for reconstitution.
- Thrombosis/Thromboembolism
- Hypertension
- Extramedullary Hematopoietic Masses